Describe the Law of Conservation of Mass
A way to describe a chemical reaction using chemical formulas and other symbols. They use two objects that collide.
What Is The Law Of Conservation Of Mass About Quora
The law of conservation of mass is one of a set of known conservation laws and it determines the conditions in which each quantity is conserved.

. It seems that the mass shrinks as the paper burns. According to the law of conservation of mass the mass of the reactants must be equal to the mass of the products for a low energy thermodynamic process. Reactants products Based on the Law of Conservation of Matter describe the relationship between the mass of the reactants and the mass of the products.
To apply the law of conservation. However what is really happening is that the paper is changing form to ash carbon dioxide and water vapor. Was the Law of Conservation of mass followed.
In a chemical reaction the total number of each type of atom is the same in the reactants and in the same products. The law of conservation of mass states that in a close container when a chemical reaction occurs no mass will be lost. Law of conservation of mass.
See answer 1 Best Answer. An isolated system is one that does not interact with its surroundings. According to the law of conservation of mass the mass of the products in a chemical.
Conservation law often known as the law of conservation is a physics principle that asserts that within an isolated physical system a specific physical attribute does not change over time. Therefore the quantity of mass is conserved over time. This is the law of mass conservation.
Some students conduct an experiment to prove conservation of momentum. Describe law of conservation of mass. The law of conservation of mass states that in a closed system the mass of the system cannot change over time.
Law of conservation of mass. According to the law of conservation of mass the mass of the products in a chemical reaction must equal the mass of the reactants. Given Alka-Seltzer and a flask students design and conduct an experiment to prove the Law of Conservation of Mass.
What is Law of Conservation of Mass. Explain the significance of the law of conservation of energy. The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations.
Therefore the mass contained in that isolated system will remain constant regardless of any transformations or chemical. Look at our example of the candle in the closed room. Mass is conserved in a chemical reaction but not during a physical change.
Describe the law of conservation of mass. Total mass of reactants before reaction Total mass of the product after reaction. The law that states that mass cannot be created or destroyed in ordinary chemical and physical changes.
When a substance transforms from a liquid state to a gaseous state it loses mass. What does the law of conservation of mass say. What is stated by the law of conservation of mass.
The law of conservation of mass is a scientific law popularized and systematized by the 18th-century French chemist Antoine Lavoisier. Given vinegar and baking soda along with specific directions students prove the Law of Conservation of Mass. Though much of the wax itself.
That means that the total amount of mass wont increase or decrease. Take the example of burning a piece of paper. What is an element.
According to the law in an isolated system matter cannot be created or destroyed only changed. As the law of conservation of mass clearly states that mass cannot be created or destroyed the waste must be somewhere within the closed system in this case earth. Chemical reactions do not produce or destroy matter.
The law of conservation of mass indicates that mass cannot be created nor destroyed. Well te len of conserwfion of mesma cariton Isoleres system is not created on destroyed my Search the channel tenetowa 2. Also to know is what is law of conservation of mass with example.
In the context of the study of chemistry the law of conservation of mass says that in a chemical reaction the mass of the products equals the mass of the reactants. C The mass of the reactants is greater than the mass of the products. The reactants atoms of one or more substances simply get rearranged to form product.
The law of conservation of mass states that The mass in an isolated system can neither be created nor be destroyed but can be transformed from one form to another. What does the law of conservation of mass say about matter. It says matter cannot be created or destroyed.
B The mass of the reactants is less than the mass of the products. This law was put forth by Antoine Lavoisier in. The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations.
In a chemical reaction except for nuclear reactions as matter can change to energy mass can neither be created nor destroyed. The Law of Conservation of Mass Matter is anything that has mass and takes up space. Why must you balance chemical equations.
The law of conservation of mass states that matter cannot be created nor destroyed only its form changed. In physics and chemistry the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy the mass of the system must remain constant over time as the systems mass cannot change so quantity can neither be added nor be removed. A The mass of the reactants equals the mass of the products.
Matter can change form through physical and chemical changes but through any of these changes matter is conserved. Law of Conservation of Mass states that matter can neither be created nor destroyed. It includes molecules atoms fundamental particles and any substance that these particles make up.
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law of conservation of mass the mass of the products in a chemical. In your own words describe the law of conservation of mass and how this law is used when writing and - balancing chemical equations.
Balancing Chemical Reactions Lab wonen Prior Knowledge Questions 1. The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations.
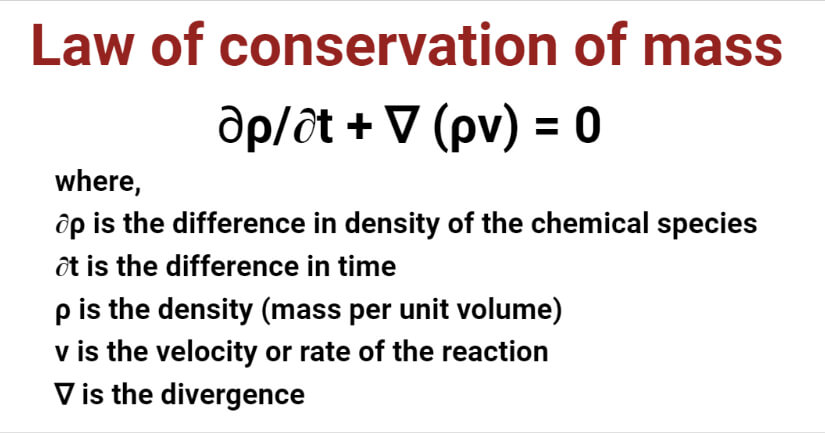
Law Of Conservation Of Mass Definition Formula Equation Examples

Comments
Post a Comment